Disclaimer to the Online Edition
This Manual has been designed for use in the NICU at London Health Sciences Centre (LHSC), London, Ontario, Canada, and represents clinical practice at this institution. The information contained within the Manual may not be applicable to other centres. If users of this Manual are not familiar with a drug, it is recommended that the official monograph be consulted before it is prescribed and administered. Any user of this information is advised that the contributors, Editor and LHSC are not responsible for any errors or omissions, and / or any consequences arising from the use of the information in this Manual.
Chloral Hydrate
Indication
- For use as a short-term sedative and hypnotic
Pharmacology
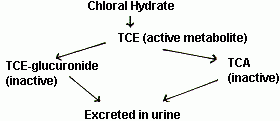
- Chloral hydrate is well absorbed from the GI tract following oral or rectal administration and is widely distributed in the body. It is rapidly metabolized in the liver and in erythrocytes by alcohol dehydrogenase to its major metabolite, trichlorethanol (TCE). TCE is responsible for most of the sedative-hypnotic effects of the drug. TCE is further conjugated to TCE-glucuronide or converted to trichloroacetic acid (TCA), both of which are believed to be inactive and are excreted in the urine
- There have been at least 2 reports (110,111) of infants with substantially elevated blood levels of TCE (250 and 72.8 mg/L) (therapeutic level for adults is reported as 10 mg/mL). The infants received 50 mg/kg q6h for 4 days, and 165 mg/kg over 12 hours, respectively. Both of these infants exhibited lethargy, decreased deep tendon reflexes, and a need for increased ventilatory support. In one of these reports(110) the authors postulated that the infant suffered chloral hydrate-induced myocardial dysfunction
- The metabolism of chloral hydrate may interfere with bilirubin metabolism, possibly resulting in elevated serum levels of unconjugated bilirubin. Therefore, monitor levels of indirect bilirubin closely
Side Effects
- The most frequently reported side effect is gastric irritation (vomiting, diarrhea) - this may be minimized by diluting the oral solution with water or feeds
- Since chloral hydrate has no analgesic properties, excitement rather than sedation may result in infants with pain
- Skin eruptions, parenchymatous renal damage or gastritis may develop following prolonged administration
- Respiratory depression, hypotension, arrhythmias and myocardial depression have all been reported
- Tolerance may develop by the second week of continued therapy
- Chloral hydrate is CONTRAINDICATED in infants with hepatic or renal impairment, gastritis or ulcers, or severe cardiac disease
- In adults, physical dependence can occur with prolonged use of large doses. Symptoms include lethargy and tremulousness. In such situations slow withdrawal of the drug is recommended. This may have implications for neonates who receive chloral hydrate for extended periods of time (I.E. weaning slowly should be considered)
- In adult patients, who have received chloral hydrate and IV furosemide, a reaction characterized by diaphoresis, flushes and variable blood pressure has been reported
Nursing Implications
- Dilute oral solution with water or feeds to minimize gastric irritation
- Closely monitor HR, BP, respirations, neurologic status
- Assess infant's response and evaluate need for continued therapy (intolerance of handling, agitation, restlessness)
- Check stools and gastric aspirate for occult blood
- Monitor liver and kidney function
Dose
- Up to 25 mg/kg po (or per rectum) q6h prn
- In view of the possibility of toxic metabolite accumulation and the lack of critical evaluation of this drug in neonates, we recommend that it not be used for extended routine sedation in neonates
- For sedation prior to a procedure (eg. EEG, CT) 50-75 mg/kg po or per rectum: sleep follows within 30-45 minutes
Supplied
- 100 mg/mL oral liquid
References
- McEvoy G K (ed): AHFS Drug Information, American Society of Hospital Pharmacists, 1991.
- Roberts, RJ: Drug Therapy in Infants, W.B. Saunders, Toronto, 1984.
- Pagliaro LA and Pagliaro AM (ed): Problems in Pediatric Drug Therapy, 1987, Drug Intelligence Publ Inc, Hamilton, Illinois.
- Gomella TL (Ed): Neonatology - Management, Procedures, On-Call Problems, Diseases, Drugs, 1992, Appleton and Lange, Norwalk, Connecticut.
- Milan EM and McFeely EJ: Memory Bank for Neonatal Drugs, 1990, Williams and Wilkins, Baltimore.
- Reimche LD, SanKaran K, Hindmarsh KW et al: Chloral hydrate sedation in neonates and infants - clinical and pharmacologic considerations, Developmental Pharmacology and Therapeutics 1989; 12: 57-64.
- Anyebuno MA and Rosenfeld CR: Chloral hydrate toxicity in a term infant, Dev Pharmacol Ther 1991: 17:116-120.
- Laptook AR and Rosenbeld CR: Chloral hydrate toxicity in a preterm infant, Pediatric Pharmacology 1984; 4:161-165.
